Submitted by Gemma Williams*(1)
(1) Gemma Williams is Director of Health Writers SLU and specialist in eating disorders in athletes and exercise psychology. Her research focuses mainly on binge eating disorder, sports nutrition and strength training.
*Corresponding Author:
Gemma Williams, BSc
Health Writers S.L.U.
Velazquez, 59 28001 Madrid
Spain
gemma.williams07@outlook.com
+34 910 846 641
ABSTRACT
Prevalence rates binge eating disorder (BED) among athletes are thought to be considerably higher than that of the general population due to strict requirements for weight and performance gains related to body composition. Traditional models of BED are based on the general population and are therefore unlikely to fully account for the development of BED in athletes, who typically display significant cognitive and dietary restraint. This review explores the scientific literature relevant to the development of BED in athletes which indicates that 1) Extreme, rigid dieting practices and preoccupation with body weight and composition is a risk factor for BED; 2) Prolonged caloric restriction with or without stressors disrupts hunger and satiety cues, results in abnormalities in neurotransmitter systems and alters fronto-striatal circuitry, driving urges to binge eat; and 3) BED shares several mechanisms and behavioural traits with drug addiction. At this time, no guidelines for the management of BED in athletes exist, and current treatments do not address the individual requirements of athletes. Knowledge that BED in athletes is likely driven by caloric restriction and multiple stressors may assist coaches and athletes in preventing the onset of BED, reducing the risk of associated psychological comorbidities and alterations in metabolism.
KEYWORDS: Binge eating disorder (BED); binge eating; athlete; eating disorders; dopamine
INTRODUCTION
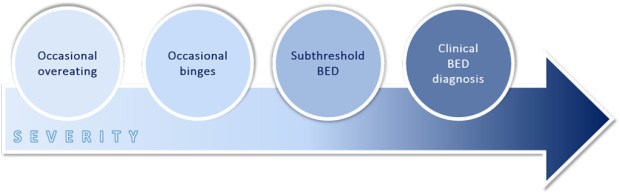
Binge eating is characterised by significant reoccurring disturbances or episodes of overeating followed by feelings of lack of control over food and distress about body shape and weight. Binge eating disorder (BED) is a clinical mental disorder, defined by the American Psychiatric Association as recurrent binge eating without compensatory weight control behaviour such as self-induced vomiting, diuretic use and excessive levels of physical activity (1). The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) states that to qualify as a BED diagnosis, binge eating episodes should occur, on average, at least once a week for 3 months (1). However, numerous clinicians and scientists subscribe to the idea of a binge eating continuum, where mild and intermittent “loss-of-control” eating develops into episodic binges which tend to become more compulsive and more frequent over time, developing into “subthreshold” BED and eventually resulting in a diagnosis of BED (Figure 1) (2).
Figure 1. The binge eating continuum: a downward escalation of overeating to binge eating disorder
Adapted from Davis (2013) (2)
Though BED is significantly associated with obesity, individuals who experience a heightened external or internal pressure for weight loss are also at risk of developing the disorder (3). As such, individuals who must adhere to strict requirements for weight and body composition, such as athletes and members of the military, have been identified as high-risk groups for BED (3). In order to avoid progression from “loss-of-control” eating to the clinical phase of BED, it is extremely important to recognise the problem early. This involves identifying risk behaviours that precede the development of BED in high-risk populations for early diagnosis and control. Therefore, the purpose of this critical research review is to investigate the current literature on BED, identify potential mechanisms of how the sports environment contributes to its development, and discuss critical information for the prevention of BED in athletes.
INCIDENCE OF BINGE EATING DISORDER IN THE ATHLETIC POPULATION
Multiple studies show that athletes report a higher incidence of eating disorders than non-athletes (4-6). According to data from the WHO World Mental Health Surveys, the prevalence of BED is 1.9% in the general population (7), with a significantly higher incidence in men compared to other eating disorders (40% for BED vs 25% for anorexia nervosa [AN] and bulimia nervosa [BN]) (8). While there has been very little research on the incidence rate and role of BED amongst athletes, existing evidence suggests that rates are much higher than that seen in the general population. Furthermore, rates in male athletes appear to be equal or even greater than that seen in female athletes. In a large-scale study of 1,445 Division I NCAA student athletes, 10% of the females reported binge eating on at least a weekly basis and 13.02% of males reported binge eating at least once a week (9). Additionally, individual studies investigating the incidence of binge eating in male bodybuilders and male rowers reported rates of 46% and 12%, respectively (10-12).
BINGE EATING DISORDER CORRELATES AND RISK FACTORS
Multiple factors have been linked to increased risk of EDs among athletes, including social pressures to adhere to the ideal body, perceived norm of sport body appearance, and perceived performance gains from achieving low levels of body fat while retaining lean body mass (13). As such, energy restriction methods are common practices amongst a number of physique sports (i.e. bodybuilders and figure competitors), combat sports (i.e. judo and wrestling competitors), aesthetic sports (i.e. gymnasts and figure skaters), and endurance sports (i.e. long distance runners) (6, 14). Furthermore, traits which enable athletes to compete and to succeed, such as determination, perfectionism, obsession, and competitiveness, also confer ED risk and can lead to body image dissatisfaction (15).
BED represents a significant health concern among athletes due to its association with significant psychiatric comorbidity, which is comparable to that of BN and AN and independent of body mass index (BMI) (1). The most common comorbid disorders include bipolar disorders, depressive disorders, anxiety disorders, and, to a lesser degree, substance use disorders (1, 16, 17). BED also has elevated risk for medical comorbidities such as metabolic and cardiovascular diseases, however, it must be noted that the majority of studies include overweight or obese subjects and therefore many associations are likely due to BMI (17).
LACK OF BINGE EATING DISORDER INTERVENTIONS IN ATHLETES
Until 2013, BED was categorised as an eating disorder not otherwise specified (EDNOS), and as such, the 2008 National Athletic Trainers’ Association Position Statement on preventing, detecting, and managing disordered eating in athletes does not provide guidelines for its management (18). In the general population, cognitive-behavioural therapy (CBT) is considered the treatment of choice for BED and has been demonstrated to be successful in reducing binging behaviours and improving psychosocial functioning through promoting regular eating patterns and moderate dietary restraint (19). However, since the majority of BED interventions have been developed and tested in the general population, the individual requirements of athletes are not catered for. Interventions that prevent, detect and manage BED in athletes while taking into account body composition and strenuous physical exercise requirements therefore represent an unmet need.
BINGE EATING DISORDER AS AN ADDICTIVE BEHAVIOUR
Behavioural parallels in binge eating and substance abuse
The causes of all eating disorders are multifactorial and include numerous and often interacting biological, psychological and sociocultural risk factors (20). In recent years, however, a strong link between substance addiction and BED has become apparent, with numerous studies demonstrating similarities in dysfunctional impulsive and compulsive behaviours, neurochemical profiles and brain structures of substance-dependent and binge-eating individuals (1, 2, 21). This is reflected in the DSM-V psychiatric classification scheme which states that addicted individuals experience a loss of control of food or drugs of abuse and continue use despite negative social and psychological consequences (Table 1) (1, 21).
Table 1. DSM-IV-TR definitions of substance dependence and binge eating disorder (1, 21)
| Comorbid symptom | Substance use disorder | Binge eating disorder |
|---|---|---|
| Escalated use | The substance is taken in larger amounts or over a longer period than originally intended |
Eating large amounts of food when not feeling physically hungry and eating more rapidly than normal |
| Loss of control | There may be a persistent desire or effort to cut down or regulate substance use, with multiple unsuccessful efforts to,decrease or discontinue use |
A sense of lack of control during the episodes, e.g., a feeling that one can’t stop eating or control what or how much one is eating |
| Social consequences | Important social, occupational, or recreational activities are given up or reduced because of use |
Eating alone because of being embarrassed by how much one is eating |
| Psychological consequences | The substance use is continued despite knowledge of having a persistent physical or psychological problem that is likely to have been caused or exacerbated by the substance |
Feeling disgusted with oneself, depressed, or very guilty after overeating; marked distress during and after binge eating; eating until feeling uncomfortably full |
Food addiction model of binge eating disorder
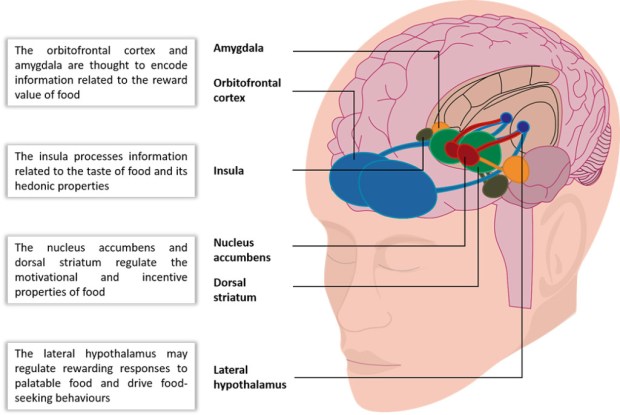
In addition to dysfunctional impulsive and compulsive behaviours, binge eating has been demonstrated to stimulate the brain’s reward and pleasure centres in a similar way to addictive drugs (2). Specifically, the consumption of processed foods with high concentrations of sugar and other refined sweeteners, refined carbohydrates, fat and salt (typical “binge” foods (20)) has been shown to directly affect the dopamine, endogenous opioid, and other neurotransmitter systems, which work in tandem to reinforce the pleasurable and rewarding properties of a stimulus (Figure 2) (22-24).
Figure 2. Areas of the brain activated in response to palatable food or food-associated cues.
Adapted from Kenney 2012 (25)
For the sake of clarity, not all interconnections between these structures are shown
Based on these findings, an increasing number of scientists and clinicians support addiction conceptualisations of BED (2, 21). The two main hypotheses in the literature are described below.
1. The “reward deficiency” hypothesis
Some authors have proposed that proposed that diets persistently high in fat and sugar down-regulate dopamine receptors due to prolonged stimulation, blunting the response to reward receipt (2, 26). The individual then requires greater exposure to rewarding stimuli (palatable food) to achieve the same level of pleasure previously experienced, which can result in an escalation of consumption and a vicious cycle of craving and compulsive overeating. This phenomenon is also seen in addictive drugs, where chronic exposure results in a diminished response so that more, or more frequent, exposure is required to elicit the strength of the initial reaction (21).
2. The “limited access” hypothesis
In another model, intermittently restricting access to highly palatable foods results in increased stimulation of the reward system when high fat and sugar foods are ingested, which in turn drives overeating and susceptibility to binge eating (2). This paradigm is one of the most rigorously tested hypotheses to explain BED, and is based on relevant DSM-IV criteria for BED (27).
However, both the “reward deficiency” and “limited access” hypotheses, which are based on either constant or intermittent access to hyperpalatable food, are unlikely to fully account for the development of BED in the athletes, who typically displays significant cognitive and dietary restraint. While the limited access model may, in part, account for the development of BED in some athletes, it is likely that additional mechanisms are at work.
Model of binge eating disorder in athletes
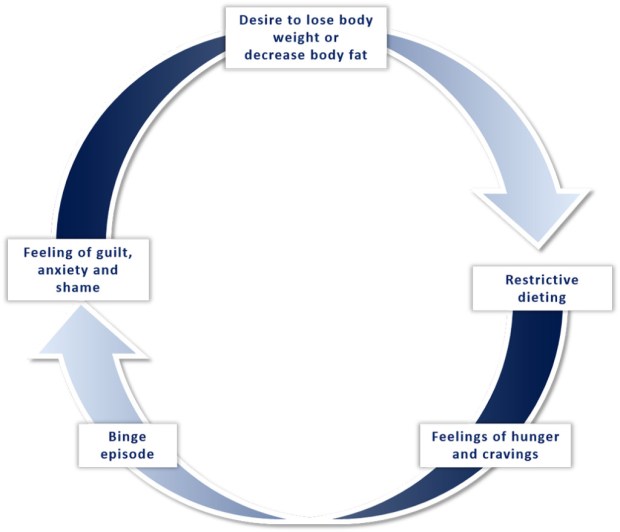
We propose that the root of binge eating behaviour in athletes may be rooted to extreme and rigid dieting practices and preoccupation with body weight and composition. This ‘dietary-induced’ model of binge eating, in which periods of calorie restriction followed by intermittent access to palatable food prompts significant binges, has previously been reported in the literature (20, 21). In a 5-Year prospective study of 496 adolescent girls, periods of marked caloric restriction and fasting (going without eating for 24 hours for weight control) was found to be a predictor of recurrent binge eating (28). This phenomenon has also been well documented in animal studies, where rats given sporadic access to foods with a high fat and sugar content increased their consumption following a fast compared to a control group where rats had access to the same palatable foods every day of the week (21). The rats given sporadic access to palatable food had overall greater consumption than the control group, ingesting up to 58% of their daily calories within the first hour of access (21).
In this model, prolonged caloric restriction disrupts hunger and satiety cues, driving urges to eat, which the individual interprets as a lack of self-control and/or “failure”. This causes the individual to abandon their efforts to restrict their eating, resulting in a binge eating episode (commonly known as “all or nothing” thinking). After binging, the individual experiences extreme concern about their inability to control their diet, as well as anxiety regarding body weight and composition. This prompts further dietary restraint, which consequently increases the risk of further bingeing. This viscous cycle is shown in Figure 3.
Figure 3. Explanatory model of the binge–restrict cycle
Mechanisms underlying binge eating disorder in athletes
1. Caloric restriction
During periods of prolonged caloric restriction, a practice commonly seen in athletes who aim to lose body fat, depletion of energy stores drives the hypothalamus to alter levels of circulating hormones (29). Studies involving energy restriction report decreases in leptin, insulin, testosterone, and thyroid hormones, as well as increases in ghrelin and cortisol (14, 30, 31). Numerous studies have demonstrated that these changes in the metabolic state can strongly affect ingestive behaviour (caloric intake) by increasing feelings of hunger and modulating the hedonic effects of food and food-related stimuli. For example, blood leptin decline is interpreted by the ventromedial hypothalamus as starvation, which drives increased gastric secretion of ghrelin, promoting feelings of hunger (32). Low levels of leptin and insulin increase sweet tasting abilities and increase the capacity to smell (33). Peripheral taste function also appears to be modulated by other agents that reflect nutrient availability, including endocannabinoids, glucagon-like peptide-1 (GLP-1) and vasoactive intestinal peptide (VIP). In other words, these agents drive a metabolic need for food by perceptually enhancing its sweet properties (33). Additionally, acute periods of marked caloric restriction have also been shown to deplete tryptophan, an amino acid precursor of serotonin, which may increase the drive to eat high carbohydrate food to restore baseline levels (28). This is consistent with the high sugar and fatty foods typically ingested during a binge episode (20).
2. Increased reinforcing properties of palatable food
As well as increasing urges to eat, periods of food deprivation or restriction has been shown to increase the rewarding properties of palatable food when it is finally eaten (33). This phenomenon is thought to be due to increased dopamine receptor sensitisation and increased stratial dopamine signalling with occurs following a period of calorie restriction (20). This increased activation of the reward pathway in response to food during caloric deficits (particularly palatable food, which generally has higher caloric value) serves to enhance pleasure and motivation to seek out food, ensuring survival (2). It is worth noting that in animal models, these neuroadaptive alterations under restricted feeding conditions occur without significant changes in body weight (i.e. the animals do not gain fat) (20). Since normal food intake is reduced after binges and weight gain does not occur, homeostatic mechanisms are preserved. This paradigm has good clinical validity for the athletic population who attempt to restrict their consumption of sweet and fatty foods, and then typically binge on the very foods they have unsuccessfully tried to avoid.
Periods of food deprivation or restriction has also been shown to alter anticipatory behaviours in response to palatable food stimuli, such as advertisements, mood, and setting. Studies have also shown that BED is associated with the development of hyper-responsivity of certain brain regions that modulate the system’s response to appetitive stimuli, such as advertisements, mood, and setting (34). These brain regions involved include the orbitofrontal cortex (OFC), a region involved in impulse control and decision-making processes, as well as the amygdala and ventral striatum, structures critical for triggering somatic states.
In a study of non-obese healthy subjects who had fasted overnight and skipped breakfast, participants viewed pictures of high-calorie versus low-calorie foods (35). Individuals viewing the high-calorie foods demonstrated increased neural activity in reward-related areas such as the orbitofrontal cortex, ventral striatum, amygdala, and anterior insula. These increases were positively correlated with subjective ‘liking’ of the foods represented by the images. In a separate study, obese and lean individuals with BED and healthy controls who had fasted overnight were exposed to visuals of high-caloric food (34). Both obese and lean individuals with BED demonstrated stronger OFC responses and enhanced reward sensitivity while viewing food pictures compared to controls (34). In other words, greater activity in the OFC during exposure to pictures of food and other food-related cues causes higher reward sensitivity, making individuals hypersensitive to the motivational and rewarding properties of food. Larger brain activation differences in the amygdala and ventral striatum have also been found in individuals with BED compared to weight-matched controls, suggesting a greater motivational sensitivity to, and visual processing of, palatable food cues in individuals with BED (36). Together, these findings suggest that in fasting states, a signal conveying “caloric need” modulates the hedonic value of specific familiar foods, with higher fat and sugar foods preferred over lower calorie options.
3. Palatable food withdrawal
In addition to enhanced hedonic properties of high calorie food, intermittent dietary restriction is also associated with periods of negative withdrawal symptoms, including depressed and irritable moods, and cravings for carbohydrates and fatty foods (2, 37). These unpleasant withdrawal symptoms motivate the binge eater to consume more high fat and sugar food to alleviate the unpleasant symptoms, similar to that seen in drug addiction (2, 37). The return to palatable food relieves the discomfort of withdrawal, thereby maintaining the binge eating behaviour through a negative reinforcement process. This has also been demonstrated in animal models of binge eating, where rats display evidence of withdrawal after several weeks of binging patterns, suggesting physical dependence has developed via changes in neurotransmitter systems, similar to drugs of abuse (27). This is consistent with findings that highly processed foods are capable of triggering cravings (38, 39).
4. External events and associated mood changes
Hebebrand et al. (2014) (40) and Hagan et al. (2002) (41) have proposed a “diet and stress” model of BED, where cycles of food restriction and refeeding with palatable food are accompanied by environmental, physiological and/or psychological stressors, representing a form of stress-evoked eating. Over time, these acute stress-triggered episodes of binge eating lead to neurobiological alterations in complex central regulatory systems related to addictive behaviours. This has been demonstrated in animal models, where acute stressors (electrical footshocks) paired with cycles of food restriction and refeeding with palatable food (also a physiological stressor) caused rats to consume twice the normal amount of said food (42). A connection between stress and binge-type eating has also been reported in humans, with several studies reporting stress, depressed mood, anger, and irritability as a precursor to binge-eating episodes and compulsive eating as a way to regulate mood (37, 43-45). This may be particularly relevant to the athletic population who may be under significant stress due to strenuous and/or excessive physical training, caloric restriction, competition pressures, lack of recreational opportunities, financial or work pressures and social issues and excessive travel (46). Indeed, studies have demonstrated that bodybuilding among men is associated with an increased risk of body dissatisfaction, weight and shape preoccupation, and pathological eating behaviours, all of which can contribute to stress (10, 47).
4. Action-to-habit devolution
Altered dopamine receptor sensitisation and stratial dopamine signalling, which results in enhanced reinforcing properties of food, is also associated with disruption of brain circuits that are involved in inhibitory control and impulsive behaviour, such as connections between the OFC and limbic structures (34). It has been suggested that disruption of these circuits can result in impulsivity and compulsive food intake (and compulsive drug intake in addiction) (34).
Recent studies have shown that exposure to high-calorie food cues elicited hyper-responsivity in brain regions involved in reward and action planning compared to weight-matched controls (48, 49). Importantly, the magnitude of these responses increased as the binge-eating symptoms increased in frequency (48). Altered processing in this area is consistent with biobehavioral findings in addictive disorders, with both food and drug-related cues causing an exaggerated response in individuals who have developed tolerance to drugs and palatable foods, respectively (21, 34). This contributes to emerging evidence that specific consumption patterns of palatable foods may produce behaviours and brain changes similar to those observed in drug addiction (50).
In this way, Smith et al (21) proposed applying the action-to-habit theory of drug addiction to BED, where an increase in impulse-driven, hedonically motivated actions devolve to compulsive, habit-driven through a process of signalling transfer in the brain. The authors suggest that overconsumption of palatable foods could initiate a similar devolution from goal-directed to habitual behaviour. In other words, the consumption of palatable foods becomes less pleasurable and instead transfers to a compulsive response triggered by certain food-related cues (21).
CONSEQUENCES OF BINGE EATING DISORDER IN ATHLETES
A strong association exists between the severity of BED and the presence of psychological problems such as bipolar disorders, depressive disorders, anxiety disorders, and, to a lesser degree, substance use disorders (1). Consequently, it is frequently associated with low self-esteem and decreased quality of life (1, 19, 51-54), and likely to negatively affect physical fitness, sport performance, personal relationships and overall productivity (55).
Studies have also shown that people who are diagnosed with BED are at greater risk of metabolic and cardiovascular diseases. While the majority of the research had been conducted in overweight or obese individuals with BED who are already at greater risk of developing these conditions, one study found that BED may confer a risk of components of the metabolic syndrome over and above the risk attributable to obesity alone (56). It is thought that the rapid consumption of large numbers of calories in one sitting and the resulting inflammatory or oxidative stress may have mediating effects in individuals with BED (56).
Furthermore, a recent analysis of data from the literature suggests that weight cycling resulting from repetitive intentional weight loss in normal weight population groups is more strongly associated with risks for metabolic and cardiovascular diseases compared with groups who do not diet (6). While this study did not include individuals with BED, repetitive attempts to lose weight or fat as well as weight cycling is characteristic of the disorder, and therefore the results could indeed be representative.
Besides psychiatric and metabolic comorbidities, BED may also drive fat regain and fat “overshooting”, a process recently described by Dulloo et al. (57) where a disproportionate rate of fat relative to lean tissue is recovered following a period of calorie restriction. Interestingly, those who are lean, rather those who are overweight or obese, seem to be most prone to the impact of fat overshooting. This can also be seen in human and animal studies of refeeding after caloric restriction (58), and repeated cycles of weight loss and regain in athletes in sports with weight classes has been shown to cause long-term weight gain (14). According to these authors, repeated dieting and weight cycling would increase the risks for trajectories from leanness to fatness, a process that would have serious implications for the athlete. However, it must be noted that these studies did not include individuals with BED.
LIMITATIONS
Although there is a substantial body of research related to BED pathology, the majority of studies have utilised animal models or subjects that are sedentary and overweight or obese. Accordingly, the current article is limited by the need to apply this data to an athletic population. It is also important to highlight the lack of research on the effect of physical exercise on the development of BED, which has been shown to be a strong risk factor in other eating disorders (15). Finally, in any model of addiction it is fundamentally important to recognise the great variability in an individual´s susceptibility to developing full-scale symptoms of dependence and loss of control over their food intake (2).
PRACTICAL IMPLICATIONS AND CONCLUSION
The information collated in this review has several practical implications:
1. Both athletes and their coaches should be a target for education about the risk behaviours that drive BED, the associated physical and psychosocial health consequences and healthy eating and reasonable training practices. They should also be vigilant about weight fluctuations and unhealthy eating habits.
2. Knowledge that BED in athletes is likely driven by caloric restriction may assist coaches and athletes in preventing the onset of BED symptoms by avoiding very low calorie or restrictive diets in susceptible individuals. Instead, a moderate calorie deficit and limited cardiovascular activity should be employed.
3. Early identification and treatment of BED should be of the highest priority. As such, if an athlete displays any signs of suffering from BED, they should be promptly referred to an eating disorder specialist for assessment and any necessary treatment. For athletes who engage in occasional binges, it can be helpful to isolate the specific factors that appear to trigger them.
Due to the seriousness of BED and its frequent appearance among athletes, it is extremely important to recognise the problem early, thus avoiding progression to the clinical phase of the disease.
ACKNOWLEDGMENTS
None
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington DAP, Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. 2013, Washington, DC: APA Press.
2. Davis C. (2013). From passive overeating to “food addiction”: a spectrum of compulsion and severity. ISRN Obes, 2013, 435027.
3. Tanofsky-Kraff M, Bulik CM, Marcus MD, Striegel RH, Wilfley DE, Wonderlich SA, et al. (2013). Binge eating disorder: the next generation of research. Int J Eat Disord, 46(3), 193-207.
4. Currie A. (2010). Sport and eating disorders – understanding and managing the risks. Asian J Sports Med, 1(2), 63-8.
5. Dick RW. (1991). Eating Disorders in NCAA Athletic Programs. Athletic Training, 26, 136-140.
6. Montani JP, Schutz Y, Dulloo AG. (2015). Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev, 16 Suppl 1, 7-18.
7. Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, et al. (2013). The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry, 73(9), 904-14.
8. Harris M, Cumella EJ. (2006). Eating disorders across the life span. J Psychosoc Nurs Ment Health Serv, 44(4), 20-6.
9. Johnson C, Powers PS, Dick R. (1999). Athletes and eating disorders: the National Collegiate Athletic Association study. Int J Eat Disord, 26(2), 179-88.
10. Andersen RE, Barlett SJ, Morgan GD, Brownell KD. (1995). Weight loss, psychological, and nutritional patterns in competitive male body builders. Int J Eat Disord, 18(1), 49-57.
11. Thiel A, Gottfried H, Hesse FW. (1993). Subclinical eating disorders in male athletes. A study of the low weight category in rowers and wrestlers. Acta Psychiatr Scand, 88(4), 259-65.
12. Sykora C, Grilo CM, Wilfley DE, Brownell KD. (1993). Eating, weight, and dieting disturbances in male and female lightweight and heavyweight rowers. Int J Eat Disord, 14(2), 203-11.
13. Sundgot-Borgen J, Torstveit MK. (2010). Aspects of disordered eating continuum in elite high-intensity sports. Scand J Med Sci Sports, 20 Suppl 2, 112-21.
14. Trexler ET, Smith-Ryan AE, Norton LE. (2014). Metabolic adaptation to weight loss: implications for the athlete. J Int Soc Sports Nutr, 11(1), 7.
15. Goltz FR, Stenzel LM, Schneider CD. (2013). Disordered eating behaviors and body image in male athletes. Rev Bras Psiquiatr, 35(3), 237-42.
16. Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, Laird NM, et al. (2008). Co-occurrence of binge eating disorder with psychiatric and medical disorders. J Clin Psychiatry, 69(2), 266-73.
17. McElroy SL, Guerdjikova AI, Mori N, O’Melia AM. (2012). Pharmacological management of binge eating disorder: current and emerging treatment options. Ther Clin Risk Manag, 8, 219-41.
18. Bonci CM, Bonci LJ, Granger LR, Johnson CL, Malina RM, Milne LW, et al. (2008). National athletic trainers’ association position statement: preventing, detecting, and managing disordered eating in athletes. J Athl Train, 43(1), 80-108.
19. Dunn EC, Neighbors C, Larimer ME. (2006). Motivational enhancement therapy and self-help treatment for binge eaters. Psychol Addict Behav, 20(1), 44-52.
20. Bello NT, Hajnal A. (2010). Dopamine and binge eating behaviors. Pharmacol Biochem Behav, 97(1), 25-33.
21. Smith DG, Robbins TW. (2013). The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry, 73(9), 804-10.
22. Cocores JA, Gold MS. (2009). The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med Hypotheses, 73(6), 892-9.
23. Ifland JR, Preuss HG, Marcus MT, Rourke KM, Taylor WC, Burau K, et al. (2009). Refined food addiction: a classic substance use disorder. Med Hypotheses, 72(5), 518-26.
24. Gearhardt AN, Davis C, Kuschner R, Brownell KD. (2011). The addiction potential of hyperpalatable foods. Curr Drug Abuse Rev, 4(3), 140-5.
25. Kenny PJ. (2011). Reward mechanisms in obesity: new insights and future directions. Neuron, 69(4), 664-79.
26. Alsio J, Olszewski PK, Levine AS, Schioth HB. (2012). Feed-forward mechanisms: addiction-like behavioral and molecular adaptations in overeating. Front Neuroendocrinol, 33(2), 127-39.
27. Babbs RK, Wojnicki FH, Corwin RL. (2012). Assessing binge eating. An analysis of data previously collected in bingeing rats. Appetite, 59(2), 478-82.
28. Stice E, Davis K, Miller NP, Marti CN. (2008). Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol, 117(4), 941-6.
29. Guyton AC, Hall JE, Dietary balances; regulation of feeding; obesity and starvation; vitamins and minerals. In Textbook of medical physiology (11th ed). 2006, Philadelphia: Saunders.
30. Geliebter A, Gluck ME, Hashim SA. (2005). Plasma ghrelin concentrations are lower in binge-eating disorder. J Nutr, 135(5), 1326-30.
31. McLean JA, Barr SI, Prior JC. (2001). Cognitive dietary restraint is associated with higher urinary cortisol excretion in healthy premenopausal women. Am J Clin Nutr, 73(1), 7-12.
32. Lustig RH. (2001). The neuroendocrinology of childhood obesity. Pediatr Clin North Am, 48(4), 909-30.
33. Berthoud HR. (2011). Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol, 21(6), 888-96.
34. Schienle A, Schafer A, Hermann A, Vaitl D. (2009). Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry, 65(8), 654-61.
35. Goldstone AP, Prechtl de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. (2009). Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci, 30(8), 1625-35.
36. Weygandt M, Schaefer A, Schienle A, Haynes JD. (2012). Diagnosing different binge-eating disorders based on reward-related brain activation patterns. Hum Brain Mapp, 33(9), 2135-46.
37. Avena NM, Bocarsly ME. (2012). Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology, 63(1), 87-96.
38. Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. (2004). Images of desire: food-craving activation during fMRI. Neuroimage, 23(4), 1486-93.
39. White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. (2002). Development and validation of the food-craving inventory. Obes Res, 10(2), 107-14.
40. Hebebrand J, Albayrak O, Adan R, Antel J, Dieguez C, de Jong J, et al. (2014). “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci Biobehav Rev, 47, 295-306.
41. Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. (2002). A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav, 77(1), 45-54.
42. Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. (2005). Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci, 119(5), 1207-14.
43. Grilo CM, White MA, Masheb RM. (2009). DSM-IV psychiatric disorder comorbidity and its correlates in binge eating disorder. Int J Eat Disord, 42(3), 228-34.
44. Haedt-Matt AA, Keel PK. (2011). Revisiting the affect regulation model of binge eating: a meta-analysis of studies using ecological momentary assessment. Psychol Bull, 137(4), 660-81.
45. Stein RI, Kenardy J, Wiseman CV, Dounchis JZ, Arnow BA, Wilfley DE. (2007). What’s driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. Int J Eat Disord, 40(3), 195-203.
46. Purvis D, Gonsalves S, Deuster PA. (2010). Physiological and psychological fatigue in extreme conditions: overtraining and elite athletes. PM R, 2(5), 442-50.
47. Goldfield GS, Blouin AG, Woodside DB. (2006). Body image, binge eating, and bulimia nervosa in male bodybuilders. Can J Psychiatry, 51(3), 160-8.
48. Filbey FM, Myers US, Dewitt S. (2012). Reward circuit function in high BMI individuals with compulsive overeating: similarities with addiction. Neuroimage, 63(4), 1800-6.
49. Geliebter A, Benson L, Pantazatos SP, Hirsch J, Carnell S. (2015). Greater anterior cingulate activation and connectivity in response to visual and auditory high-calorie food cues in binge eating: Preliminary findings. Appetite, 96, 195-202.
50. Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Rajita S, et al. (2013). Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring), 21(2), 367-77.
51. Cassin SE, von Ranson KM, Heng K, Brar J, Wojtowicz AE. (2008). Adapted motivational interviewing for women with binge eating disorder: a randomized controlled trial. Psychol Addict Behav, 22(3), 417-25.
52. Linde JA, Jeffery RW, Levy RL, Sherwood NE, Utter J, Pronk NP, et al. (2004). Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord, 28(3), 418-25.
53. Masheb RM, Grilo CM. (2006). Emotional overeating and its associations with eating disorder psychopathology among overweight patients with binge eating disorder. Int J Eat Disord, 39(2), 141-6.
54. Sheehan DV, Herman BK. (2015). The Psychological and Medical Factors Associated With Untreated Binge Eating Disorder. Prim Care Companion CNS Disord, 17(2).
55. El Ghoch M, Soave F, Calugi S, Dalle Grave R. (2013). Eating disorders, physical fitness and sport performance: a systematic review. Nutrients, 5(12), 5140-60.
56. Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, et al. (2010). Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr, 91(6), 1568-73.
57. Dulloo AG, Montani JP. (2015). Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: an overview. Obes Rev, 16 Suppl 1, 1-6.
58. Dulloo AG, Jacquet J. (2001). An adipose-specific control of thermogenesis in body weight regulation. Int J Obes Relat Metab Disord, 25 Suppl 5, S22-9.

