Abstract
We do not clearly understand what type and duration of recovery works best after a hard run to restore the body to peak racing condition. This study compared 72 hr of active recovery after a 5-km running performance with 72 hr of passive recovery. A sample of 9 male and 3 female runners of above-average ability completed 3 trials within 6 days. Each 5-km trial was followed by 72 hr of passive recovery (PAS) or 72 hr of active recovery (ACT), a counterbalanced protocol. The 2 initial 5-km trials constituted separate PAS and ACT baselines. Mean finishing times did not differ significantly (p = 0.17) between ACT (19:35 + 1.5 min) and baseline (19:41 + 1.7 min); nor was there significant difference (p = 0.21) between PAS (19:30 + 1.5 min) and baseline (19:34 + 1.6 min). Average heart rate for PAS (177.9 + 6.3 b/min) was significantly higher (p = 0.04) than baseline (175.4 + 6.5 b/min), but ACT average heart rate (175.9 + 6.6 b/min) was significantly lower (p = 0.02) than baseline (178.9 + 6.4 b/min). For PAS, perceived rate of exertion at ending (19.8 + 0.6) was significantly greater (p = 0.01) than baseline (19.3 + 0.9), yet for ACT, perceived rate of exertion at ending (19.6 + 0.8) did not differ significantly (p = 0.17) from baseline (19.7 + 0.7). During PAS trials, 2 individuals ran a mean 12.0 + 2.8 s slower, 2 individuals ran a mean 33.0 + 21.0 s faster, and 8 individuals ran within 5.1 + 2.5 s of their first run. During the ACT trials, 1 participant ran 13.0 s slower, 3 participants ran a mean of 34.7 + 13.5 s faster, and 8 nonresponders ran within 5.5 + 2.7 s of baseline. Results indicate that 72 hr of passive and active recovery result in similar mean 5-km performance.
Active Versus Passive Recovery in the 72 Hours After a 5-km Race
In order to improve race day performance, many runners believe, daily running is necessary, with many runs made with intense effort. However, excessive numbers of consecutive hard efforts with no allowance for sufficient recovery can lead to overtraining. Combining appropriate training frequency with adequate rest may optimize race day performance.
Previous studies have focused on recovery from long endurance races, such as marathons and ultra-marathons (Gomez et al., 2002; Martin & Coe, 1997; Noakes, 2003). Recovery from such endurance races revolves around the repairing of damaged muscle fibers and replenishing of glycogen stores (Gomez et al., 2002; Nicholas, Green, Hawkins, & Williams, 1997). However, when it comes to endurance activities of shorter duration, such as a 5-km (3.1 mi) or 10-km race, Foss and Keteyian (1998) indicate that, while muscle and liver glycogen levels may have normalized 24 hr after exercise, muscle function and performance measures may not be fully recovered.
New Zealander Jack Foster indicated a runner should take one recovery day for every mile completed in a race; Joe Henderson indicated that it may be even better to take one easy day per kilometer of racing distance (Brown & Henderson, 2002; Galloway, 1984; Henderson, 2000; Higdon, 1998; O’Conner & Wilder, 2001; Sinclair, Oglesby, & Pierpenburg, 2003). While Henderson’s and Foster’s statements about duration of recovery after a race or hard effort seem appropriate, the most effective length and type (active or passive) of recovery before resuming racing or intense training have yet to be fully determined.
Often—and despite the possibility that athletes who recover relatively longer may actually feel recovered and ready to pick up intense running efforts again—athletes worry about time off (or even lighter training) as they try to balance the need to recover with the fear of reduced fitness or diminished racing performance (Houmard & Johns, 1994; Kubukeli, Noakes, & Dennis, 2002). Active recovery, such as light concentric exercise after strenuous training and racing, is commonly practiced by distance runners, in hopes of enhancing recovery and providing the needed training stimulus to maintain fitness (Brown & Henderson, 2002; Galloway, 1984; Wigernaes, Hostmark, Kierulf, & Stromme, 2000). However, again, the intensity and duration of running needed for sufficient recovery is not well understood.
Some argue that balancing periods of complete rest with periods of active recovery enhances the recovery process, which in turn allows for a greater effort during hard training sessions and on race day (Martin & Coe, 1997; Noakes, 2003; Martin, Zoeller, Robertson, & Lephart, 1998; Wigernaes et al., 2000). However, many runners often complain of feeling “stale” if they haven’t run in a few days; it has been suggested that competitive athletes who for some reason begin to train less often may experience a loss of “feel” during exercise (Mujika et al., 2001). Engaging in active recovery, at an appropriate level of intensity, following a hard session often leads to feeling somewhat invigorated at the start of a subsequent intense effort (Martin & Coe, 1997). Mujika and colleagues (2001) showed that active recovery during a six-day taper preceding a major 800-m race allowed runners to run faster than when they had recovered passively for six days. It may be that pursuing some type of activity tends to improve state of mind prior to a race or intense training effort, which may provide a rationale for improvement (Martin & Coe, 1997; Noakes, 2003).
When a pilot study was conducted for the present research, one participant who took 72 hr of active recovery subsequently ran 90 s faster than the previous 5-km time; the other participant, who also took 72 hr of active recovery, recorded extremely similar times in the two 5-km trials. However, when two trials were instead separated by 72 hr of passive recovery, the first participant ran an identical time in each trial, while the second ran 28 s faster in the trial following 72 hr of passive recovery. This discrepancy between the two runners’ times leaves it unclear which recovery method might be more effective.
The pilot study results along with the lack of established standards as to duration of active and passive recovery, and optimal intensity of activity in the former, suggested further investigation was needed to determine if one type of recovery results in a more effective return to running than another, tending to improve performance during the next race or hard training session. Therefore, the present study compared the relative impacts on 5-km running performance of 72 hr of passive recovery and 72 hr of active recovery.
Method
Participants
Study participants were 12 well-trained male runners (n = 9) and female runners (n = 3) runners currently engaged in rigorous training. Runners from the local road running and track club, local triathlon competitors, and former high school and college competitive runners were recruited by word of mouth. Criteria for selection of participants included the following: (a) current distance-running training program in place; (b) recorded 5-km run time of 16–22 min for males or 18–24 min for females; (c) minimum current weekly average of 20–30 mi running; (d) previous completion of at least five 5-km road or track races; (e) VO2max of at least 45 ml/kg/min for females or 55 ml/kg/min for males; and (f) submitted self-report data indicating good health (e.g., questionnaire over running history, Physical Activity Readiness Questionnaire, Health Readiness Questionnaire).
Participants completed a short questionnaire describing their running background, racing history, and current training mileage. All participants were volunteers and signed an informed consent form outlining the study’s requirements and potential risks and benefits to participants.
Procedures
We assessed participants’ age, height, body weight, and body fat percentage [using a 3-site skinfold technique (Brozeck & Hanschel, 1961; Pollack, Schmidt, & Jackson, 1980)]. Participants were fitted with a Polar brand heart rate monitor and then completed a graded exercise test (GXT) to exhaustion lasting 12–18 min. VO2max, heart rate, and ratings of perceived exertion were collected every minute.
All GXTs were completed on a Quinton 640 motorized treadmill. The test began with a 2 min warm-up at 2.5 mph. Speed was increased to 5 mph for 2 min, followed by 2 min at 6 mph, 2 min at 7 mph, and 2 min at 7.5 mph; from this point on, incline was increased 2% every 2 min, until the participant reached volitional exhaustion (i.e., indicated that continued running at the required speed and grade was impossible). Once participants reached volitional exhaustion, they were instructed to cool down, continuing to run at low intensity until they felt recovered.
Approximately five days after the GXT, participants performed the initial subsequent 5-km race, between 6:30 a.m. and 7:30 a.m. (Time of day of each performance trial was held consistent throughout the study.) All 5-km trials were completed on a flat, hard-surfaced, 0.73 mi loop. Prior to each trial, participants completed a visual analogue scale before and after a 1.5 mi warm-up run, answering questions about fatigue or soreness being experienced within the quadriceps, hamstrings, gastrocnemius, lower body, and total-body muscle groups. The visual analogue scales comprised 15 cm lines on which participants marked an X to indicate fatigue or soreness, from 0 (no fatigue or soreness) to 15 (extreme fatigue or soreness). The subjective visual analogue scales evaluated participants’ status before the start of every time trial. Participants were also required to rate their perceived exertion (RPE) after the warm-up and prior to the start of each 5-km trial, to see if feelings of effort remained consistent between each trial, as well as during each lap and at the end of each performance trial.
Runners underwent a 1.5 mi warm-up prior to every 5-km performance trial (Kaufmann & Ware, 1977). Participants completed three 5-km performance trials within a 6-day period. Two 5-km performance trials were separated by 72 hr of passive recovery (PAS), and two 5-km performance trials were separated by 72 hr of active recovery (ACT), providing a counterbalanced protocol. The first and second 5-km performance trials were considered to provide separate baselines. All subjects were required to have 24 hr of passive recovery prior to the first performance trial. Passive recovery comprised a period of time in which exhausting physical activity was to be avoided; active recovery consisted of running 5 mi on a flat course on two consecutive days, at an intensity of 65%–75% of maximum heart rate. During each time trial, average heart rate (HRave) and ending RPE (RPEend) were recorded at each lap, to determine whether effort during each 5-km trial was consistent. All runners competed with runners of similar ability to simulate race day and hard-training conditions, while verbal encouragement was provided often and equally to each participant. At the end of each performance trial, each runner was instructed to complete a low-intensity, 1.5-mi cool-down run. Each trial session lasted approximately 60 min.
Statistical Analysis
Basic descriptive statistics were computed along with repeated measures of analysis of variance (ANOVA), for comparison of baseline performance trial results with results following active recovery (ACT) and passive recovery (PAS), in terms of (a) finishing times, (b) HRave, (c) RPEend, and (d) visual analogue responses concerning fatigue and soreness. All statistical comparisons were made at an alpha < 0.05. Data were expressed as the group mean ± standard deviation and as individual results.
In order to evaluate individual responses, data from each participant’s first run was compared to that from the second run, using a paired t test. The least significance group mean difference (p < 0.05) was determined, and group mean finishing time was adjusted to determine the amount of change (in seconds) needed to achieve significance. The time change between the first trial run and the adjusted trial run baseline was divided by the first trial run and expressed as either a mean number of seconds or a percentage, for both the ACT trial (8.9 s, or 0.7%) and PAS trial (7.0 s, or 0.6%). The percentages were applied to each individual baseline time, in order to determine, for both ACT and PAS conditions, how many seconds above or below the first trial time (positive or negative) the second trial time needed to be to allow a response to be quantified. Participants were then labeled nonresponders, positive responders (faster on successive trial), and negative responders (slower on successive trial).
Results
Descriptive statistics are found in Table 1. The participants were between 18 and 35 years of age, the majority between 20 and 28. All participants were trained runners or triathletes who specialized in running.
Table 1
Descriptive Statistics for 9 Male and 3 Female Study Participants
| Males | Females | Group | Males | Females | Group | |
|---|---|---|---|---|---|---|
| Age, in years | 25.6 | 22.0 | 24.7 | 5.0 | 1.0 | 4.6 |
| Height, in centimeters | 175.3 | 168.0 | 173.5 | 6.2 | 18.2 | 10.0 |
| Weight, in kilograms | 78.0 | 61.7 | 73.9 | 10.9 | 10.0 | 12.6 |
| Body fat percentage | 10.9 | 21.9 | 13.7 | 1.3 | 2.0 | 5.1 |
| VO2max (ml/kg/min) |
63.3 | 59.7 | 62.4 | 5.0 | 7.9 | 5.6 |
| Prestudy 5-km personal best, in minutes |
18:57 | 21:31 | 20:19 | 1:54 | 2:05 | 2:02 |
| Average weekly mileage | 31.7 | 30.1 | 30.5 | 7.4 | 7.7 | 7.5 |
| Days per week | 4.9 | 4.6 | 4.7 | 1.5 | 1.1 | 1.2 |
Mean finishing times, HRave, and RPEend for ACT and PAS performance trials are presented in Table 2. ACT did not differ significantly (p = 0.17) from the baseline, nor did PAS (p = 0.21). In terms of HRave, ACT was significantly lower (p = 0.02) than baseline, while PAS was significantly higher (p = 0.04) than baseline. For the ACT condition, RPEend was not significantly different (p = 0.17) from the baseline, but for the PAS condition it was significantly difference (p = 0.01) from baseline.
Table 2.
Mean Performance Trial Results After ACT and After PAS
Baseline
PAS
Baseline
ACT
Finish time, in minutes
19:34 + 1.60
19:30 + 1.52
19:41 + 1.66
19:35 + 1.54
Average heart rate, in b/min
175.4 + 6.5
177.9 + 6.3a
178.9 + 6.4
175.9 + 6.6 a
Rating of perceived exertion at ending
19.3 + 0.9
19.8 + 0.6 a
19.7 + 0.7
19.6 + 0.8
Note. “After ACT” indicates performance trial completed following 72 hr of active recovery; “After PAS” indicates performance trial completed following 72 hr of passive recovery
aTrial results differed significantly from those of baseline trial.
Figure 1 shows individual changes in finishing times for all ACT and PAS performance trials.
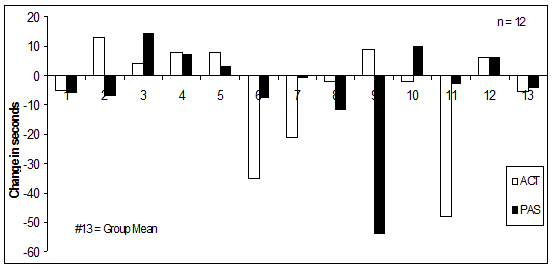
Figure 1. Changes in individual finishing times, ACT versus PAS.
A participant labeled a nonresponder had an individual time change that fell within 0.7% of baseline performance for ACT and within 0.6% of baseline performance for PAS. Positive responders and negative responders (Table 3) were those participants whose individual time change surpassed 0.7% for ACT trials or 0.6% for PAS trials. A positive responder’s time improved during the second performance trial (expressed as a negative value), while a negative responder’s time slowed during the second performance trial (expressed as a positive value).
Table 3.
Individual Performance Trial Results After ACT and After PAS
Participant
Baseline
ACT
Time
Baseline
PAS
Time
1
16:47
16:42
-5
16:42
16:36
-6
2
17:32
17:40
+8
17:25
17:32
+7
3
17:37
17:50
+13a
17.44
17:37
-7
4
18:48
18:46
-2
18:38
18:48
+10
5
19:31
19:35
+4
19:35
19:49
+14a
6
19:57
20:05
+8
20:05
20:08
+3
7
20:10
19:49
-21a
19:49
19:48
-1
8
20:37
20:35
-2
20:49
20:37
-12a
9
20:48
20:13
-35a
20:13
20:05
-8
10
21:08
21:14
+6
21:14
21:20
+6
11
21:21
21:30
+9
21:30
20:36
-54a
12
21:53
21:05
-48a
21:05
21:02
-3
M
19:41
19:35
-5.4
19:34
19:30
-4.3
Note. “After ACT” indicates performance trial completed following 72 hr of active recovery; “After PAS” indicates performance trial completed following 72 hr of passive recovery. In the change columns, a minus sign indicates a faster time, and a plus sign indicates a slower time.
aParticipant labeled a responder.
Two individuals responded negatively to PAS, running a mean 12.0 + 2.8 s slower during the second PAS trial (Table 3). Two individuals responded positively to PAS, running a mean 33.0 + 21.0 s faster than baseline. Eight individuals were considered nonresponders to PAS, performing within 5.1 + 2.5 s of baseline.
One individual responded negatively to ACT, running 13 s slower during the ACT trial (Table 3). Three individuals responded positively to ACT, running a mean 34.7 + 13.5 s faster than baseline. Eight individuals were nonresponders to ACT, performing within 5.5 + 2.7 s of baseline. It is important to note that neither negative responder to PAS (Participant 3, Participant10) was in addition a negative responder to ACT (only Participant 2 was a negative responder to ACT). Furthermore, no participant was a positive responder to both PAS and ACT. (Participant 8 and Participant 9 were PAS positive responders; Participant 6, Participant 7, and Participant 11 were positive responders to ACT).
Comparing the ACT and baseline trials, there was no significant difference in results of the pre-warm-up and post-warm-up visual analogue scales of soreness and fatigue; neither was a significant difference observed between the PAS and baseline trials, in terms of soreness and fatigue (see Table 4). At the start of each trial, the participants appeared to have been fully recovered from previous exertion.
Table 4. Soreness and Fatigue Surrounding ACT Performance Trials and PAS Performance
Trials
Performance Trial | Analogue Visual Scale | Pre-Warm-up | Post-Warm-up
Soreness
Fatigue
Soreness
Fatigue
ACT
Baseline
6.5 + 1.4
6.1 + 1.1
6.4 + 0.8
6.3 + 1.2
Day 2
6.4 + 0.7
6.0 + 0.8
6.2 + 1.2
6.7 + 0.7
PAS
Baseline
5.8 + 1.3
5.9 + 0.9
6.2 + 0.6
6.3 + 1.4
Day 2
6.3 + 0.6
5.8 + 0.5
6.5 + 0.9
5.9 + 0.8
Note. The results showed no significant differences between trials. Participants appeared to be fully recovered prior to the start of each trial.
Discussion and Conclusion
How exactly a subsequent endurance performance is affected by an active recovery as compared to a passive recovery remains something of a mystery. Despite a variety of researchers’ focus on the two types’ effects on anaerobic performance (i.e., repeated short, intense efforts running or cycling), results have been equivocal (Boqdanis, Nevill, Lakomy, Graham, & Louis, 1996; Coffey, Leveritt, & Gill, 2004; Dupont & Berthoin, 2004; Spierer, Goldsmith, Baran, Hryniewicz, & Katz, 2004; Spencer, Bishop, Dawson, Goodman, & Duffield, 2006). Specific, possibly unknown effects of active and passive recovery on individual anaerobic performance tend to support the notion of individual variability: That is, what works for one athlete may not work for another. Individual variability may, then, exist among individuals in terms of their ability to recover from endurance performances like 5-km or 10-km races. Whether recovery should be active or passive has not been fully examined for distance running.
Following an endurance performance, Foss and Keteyian (1998) have noted, 24 hr of passive recovery may allow for normalization of muscle and liver glycogen, yet muscle function and performance measures may not be fully recovered; additional time, for example a 72-hr period, may be sufficient to allow optimal recovery. Running’s catabolic nature results in pain from microtears and edema (swelling) that occur within the muscle (Brown & Henderson, 2002). The damage is only addressed with sufficient passive recovery prior to further intense effort (Bosak, Bishop, & Green, 2004). Moreover, increased recovery time can reduce reflex muscle spasms and spastic conditions that accompany pain (Brown & Henderson, 2002). In the present study, no significant difference (p = 0.17) was observed between baseline and ACT or baseline and PAS. Hence, 72 hr of both active and passive recovery (Table 2, Figure 1) appeared to allow sufficient recovery, permitting most study participants to perform almost identical mean times between baseline trials and PAS and ACT trials.
Although we found no significant mean differences between PAS and baseline or ACT and baseline, it is important to focus on individual differences (Figure 1). Eight individuals, considered nonresponders to PAS, had a mean time change of positive or negative 5.2 + 2.5 s. For these nonresponders, 72 hr of passive recovery seemed sufficient for full recovery prior to the PAS trial. Two participants responded positively to PAS, with a mean 33.0 + 21.0 s faster during the second trial (Table 3). Improved performance during the PAS trial might, however, be attributable to the participants’ being better rested than they were at the time of the first trial. Participants had been encouraged to train normally and then recover passively for a day prior to the first trial; it is therefore possible that the two participants recovered better in advance of the PAS trial than in advance of the baseline trial.
Three individuals responded positively to ACT, running a mean 34.7 + 13.5 s faster during that trial than the prior one; however, eight individuals were nonresponders to ACT, having a mean time change of 5.5 + 2.7 s (Table 3). The roughly equal or slightly faster times recorded during the ACT trial might have been a result of participants’ fuller recovery over the 72-hr period prior to the ACT trial than during the single day of passive recovery preceding baseline trials.
A single participant raced slower during ACT and PAS trials, performance falling off by 13 s following a 72-hr period of active recovery (Table 3). This relative slowness during the second trial could have resulted from the intensity of two 5-mi recovery runs the participant completed on consecutive days in the 72-hr active recovery period preceding the ACT trial. While participants were instructed to run 5 mi at 65%–75% of maximum heart rate, that level of intensity might have prevented the individual’s muscle function from returning to normal (Brown & Henderson, 2002). As for the PAS trials, despite the 72-hr passive recovery period, two participants nevertheless recorded a mean time 12.0 + 2.8 s slower during the PAS performance trial. Slowing during PAS may have been a result of the “stale” feeling in leg muscles reported by Mujika et al. (2001) to affect runners who avoid exercise for some days. It has been suggested that competitive athletes who for some reason begin to train less often may experience a loss of “feel” during exercise, which can diminish subsequent performance (Mujika et al., 2001).
Average heart rate recorded for the ACT trial was significantly lower (p = 0.02) than baseline HRave, despite the similarity of mean ACT finishing times. During the PAS trial, HRave was significantly higher (p = 0.04) than baseline, even though, again, PAS and baseline mean finishing times were similar. The data for individuals show only Participant 8 and Participant10 to have run faster and recorded higher HRave during the ACT and PAS trials as compared to the baseline. Only Participant 4 ran slower and had a lower HRave during the PAS trial than during the baseline trial; during the ACT trial, four participants (3, 4, 5, and 12) ran slower and had lower HRave during the second-day performance. An assumption could be made that low HRave reflects lesser effort, since heart rate and level of intensity tend to be linearly related. But no consistent pattern of HRave and increased or decreased performance was found here for all subjects during all PAS and ACT trials.
Also relating to participants’ level of effort across all trials, we paid particular attention to RPEend and the visual analogue scales of fatigue and soreness. No significant differences (p = 0.17) were found from ACT to baseline for RPEend. In contrast, between PAS RPEend and baseline RPEend a significant difference (p = 0.01) was observed, the PAS score being higher (although a half-unit change in RPEend is potentially meaningless). Soreness and fatigue scores from the visual analogue scales did not differ significantly from baseline to ACT or baseline to PAS, indicating that runners on average tended to feel about the same prior to each 5-km trial (Table 4). Because the study yielded no clear relationship between HRave and RPEend during trials, and because no significant difference in responses were observed between administrations of the fatigue/soreness visual analogue scales, we assume that each participant completed each trial exerting similar effort.
The results of the study indicate that 72 hr of passive or active recovery constituted sufficient recovery to maintain or improve 5-km performance. Previous research does not agree on the best mode of recovery. Noakes (2003) and Henderson (2000) indicate that 72 hrs of passive recovery is potentially superior to active recovery, allowing for restoration of proper muscle function. Martin et al. (1998), however, contend that performing low-intensity aerobic exercise immediately after exercising helps improve subsequent performance. To the discussion, we add this study’s finding, for the sample as a whole, that the passive or active nature of a 72-hr recovery period made no difference in subsequent performance.
To improve race performance, frequent, intense workouts are vital; but it is also important to make optimal use of time between competitive races and strenuous training periods: recovery time. Individual variability has been said to characterize recovery, and it was definitely evident in carrying out this study. Understanding their individual responses to particular recovery protocols may aid runners in determining how often they can complete intense training runs during the week without diminishing their weekend race performance. Future research might examine whether a longer or shorter duration for recovery, passive or active, enhances running performance.
References
Boqdanis, G. C., Nevill, M. E., Lakomy, H. K., Graham, C. M., & Louis, G. (1996). Effects of active recovery on power output during repeated maximal cycling. European Journal of Applied Physiology, 74(5), 461–469.
Bosak, A., Bishop, P., & Green, M. (2004). Comparison of 5km Racing Performance after 24 and 72 Hours of Passive Recovery. Unpublished doctoral dissertation, University of Alabama.
Brown, R. L., & Henderson, J. (2002). Fitness Running (2nd ed). Champaign, IL: Human Kinetics.
Brozek, J., & Hanschel, A. (1961). Techniques for measuring body composition. Washington, DC: National Academy of Sciences.
Coffey, V., Leveritt, M., & Gill, N. (2004). Effects of recovery modality on 4-hour repeated treadmill performance and changes in physiological variables. Journal of Science and Medicine in Sport, 7(1), 1–10.
Dupont, G., & Berthoin, S. (2004). Time spent at a high percentage of VO2max for short intermittent runs: Active versus passive recovery. Canadian Journal of Applied Physiology, 29, S3–S16.
Foss, M. L., & Keteyian, S. J. (1998). Fox’s physiological basis for exercise and sport. Ann Arbor, MI: McGraw-Hill.
Galloway, J. (1984). Galloway’s book on running. Bolinas, CA: Shelter.
Gomez, A. L., Radzwich, R. J., Denegar, C. R., Volek, J. S., Rubin, M. R., Bush, J. A., Doan, B. K., Wickham, R. B., Mazzetti, S. A., Newton, R. U., French, D. N., Hakkinen, K., Ratamess, N. A., & Kramer, W. J. (2002). The effects of a 10-kilometer run on muscle strength and power. Journal of Strength and Conditioning Research, 16, 184–191.
Henderson, J. (2002). Running 101: Essentials for success. Champaign, IL: Human Kinetics.
Higdon, H. (1998). Smart Running. Emmaus, PA: Rodale Press.
Houmard, J. A., & Johns, R. A. (1994). Effects of taper on swim performance: Practical implications. Sports Medicine, 17, 224–232.
Kaufmann, D. A., & Ware, W. B. (1977). Effect of warm-up and recovery techniques on repeated running endurance. The Research Quarterly, 2, 328–332.
Kubukeli, Z. N., Noakes, D., & Dennis, S. C. (2002). Training techniques to improve endurance exercise performances. Sports Medicine, 32, 489–509.
Martin, D. E., & Coe, P. N. (1997). Better training for distance runners (2nd ed.). Champaign, IL: Human Kinetics.
Martin, N. A., Zoeller, R. F., Robertson, R. J., & Lephart, S. M. (1998). The comparative effects of sports massage, active recovery, and rest in promoting blood lactate clearance after supramaximal leg exercise. Journal of Athletic Training, 33, 30–36.
Mujika, I., Goya, A., Ruiz, E., Grijalba, A., Santisteban, J., & Padilla, S. (2001). Physiological and performance responses to a 6-day taper in middle-distance runners: Influence of training frequency. International Journal of Sports Medicine, 23, 367–373.
Noakes, T. (2003). Lore of running (4th ed.). Champaign, IL: Human Kinetics.
Nicholas, C. W., Green, P. A., Hawkins, R. D., & Williams, C. (1997). Carbohydrate intake and recovery of intermittent running capacity. International Journal of Sport Nutrition, 7, 251–260.
O’Conner, F. G., & Wilder, R. P. (2001). Textbook of running medicine. New York: McGraw-Hill.
Pollock, M. L., Schmidt, D. H., & Jackson, A. S. (1980). Measurement of cardiorespiratory fitness and body composition in the clinical setting. Comprehensive Therapy, 6, 12–27.
Sinclair, J., Olgesby, K., & Pierpenburg, C. (2003). Training to achieve peak running performance. Boulder, CO: Road Runner Sports.
Spencer, M., Bishop, D., Dawson, B., Goodman, C., & Duffield, R. (2006). Metabolism and performance in repeated cycle sprints: Active vs. passive recovery. Medicine and Science in Sports and Exercise, 38(8), 1492–1499.
Spierer, D. K., Goldsmith, R., Baran, D. A., Hryniewicz, K., & Katz, S. D. (2004). Effects of active versus passive recovery on work performed during serial supramaximal exercise tests. International Journal of Sports Medicine, 25(2), 109–114.
Wigernaes, I., Hostmark, A. T., Kierulf, P., & Stromme, S. B. (2000). Active recovery reduces the decrease in circulating white blood cells after exercise. International Journal of Sports Medicine, 21, 608–612.

